OTTAWA -- Health Canada is advising consumers that Mylan Pharmaceuticals ULC has begun a voluntary recall of certain lots of a nitroglycerin heart medication from retailers.
Some lots of the product, called Mylan-Nitro Sublingual Spray 0.4 mg Per Metered Dose, are potentially missing the dip tube, a part of the pump component. A missing dip tube could pose a problem with delivery of nitroglycerin to the patient.
Health Canada previously informed consumers about this issue Sept. 20, but the recall was delayed due to the possibility of creating a shortage of this medically necessary heart drug on the Canadian market.
Nitroglycerin is used to prevent or relieve a sudden attack of chest pain, called angina, caused by heart disease.
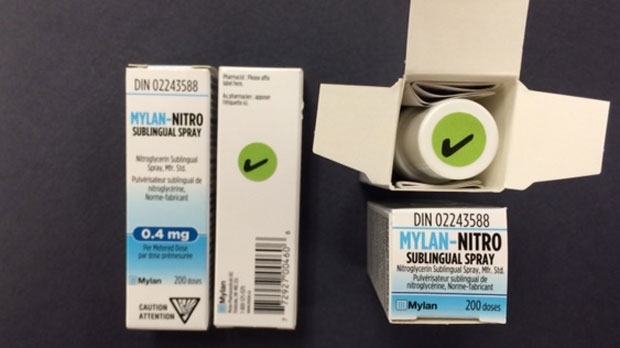
Mylan Pharmaceuticals is now releasing the product from a different lot that has been verified to include a dip tube. A checkmark sticker on the product's canister closure and carton shows the product contains the device.
To ensure ongoing supply of the nitroglycerin spray, the company is asking consumers to continue use of their current canister and to only refill their prescription once one-third of its contents -- about 70 to 75 doses -- has been used.